Soil pH: A Key to Healthy Plants and Gardens
Soil pH is one of the most important factors for keeping plants healthy and productive. It measures how acidic or alkaline the soil is and directly impacts how well plants can take in nutrients and how active the soil microbes are. For gardeners and farmers, understanding soil pH is essential for better crop yields, healthier plants, and more sustainable practices.
Table of Contents
What Is pH?
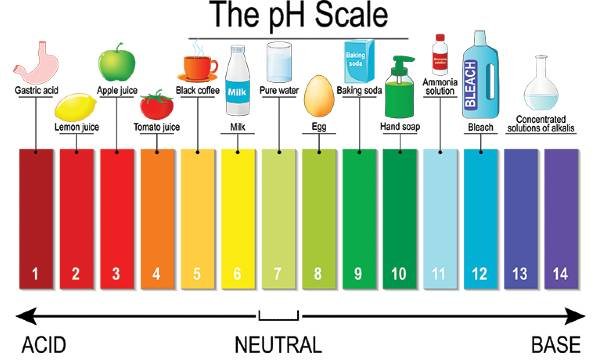
pH is a scale from 0 to 14 that measures how acidic or alkaline something is. A pH of 7 is neutral. Numbers below 7 indicate acidity, while numbers above 7 indicate alkalinity. For example, lemon juice has a pH of around 2 (very acidic), while baking soda has a pH of about 8.5 (alkaline).
In soil, pH controls the environment where plants grow. Acidic soils have more hydrogen ions, and alkaline soils have fewer. Most plants grow best in soil with a pH between 6.5 and 7 because nutrients are easiest to absorb in this range. However, some plants, like blueberries, need more acidic soil, while others, like lavender, prefer alkaline conditions.
Why Is Soil pH Important?
Soil pH affects how plants absorb nutrients. Plants rely on dissolved nutrients to grow, and pH determines how much of those nutrients are available. For example:
- Acidic soils (below pH 6): Nutrients like nitrogen and potassium are harder for plants to absorb.
- Alkaline soils (above pH 7.5): Micronutrients like iron and manganese become unavailable, which can cause yellowing leaves and poor growth.
If the soil pH isn’t right for your plants, they may show signs of nutrient deficiency even if you’ve fertilized them. For example, blueberries need acidic soil with a pH of 4.5 to 5.5. If the soil is too alkaline, they can’t absorb the nutrients they need to thrive.
Microbes and Soil pH
Soil pH also affects the activity of microbes that break down organic matter. In acidic soils, fungi are more active, while bacteria are more common in alkaline soils. These microbes help release nutrients into the soil, making them available for plants. By balancing soil pH, you can support a diverse microbial community, which improves soil health overall.
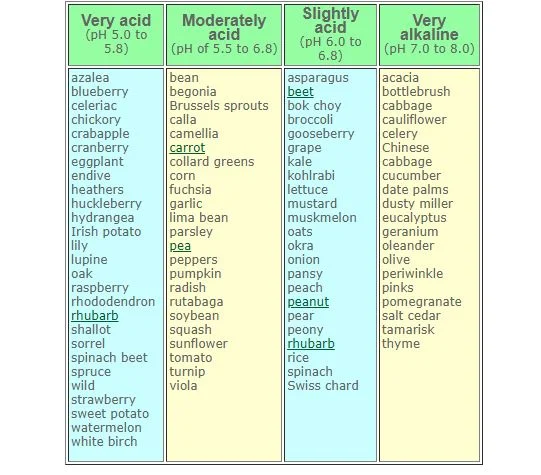
Plant Preferences for pH
The pH Scale
The pH scale isn’t linear; it’s logarithmic. This means each step on the scale represents a tenfold change in acidity or alkalinity. For example, soil with a pH of 5 is ten times more acidic than soil with a pH of 6. This is why even small changes in soil pH can have a big effect on plant health.
Example: Fixing Soil pH in Canterbury
A farmer in Canterbury, New Zealand, was struggling with poor corn yields despite adding fertilizer. A soil test showed the pH was 5.2, which was too acidic for the corn to absorb phosphorus. The farmer applied lime to raise the pH to 6.5. The next season, the corn grew much better, showing how important it is to manage soil pH.
How pH Affects Nutrients
Soil nutrients are only available to plants within certain pH ranges:
- Below pH 6: Macronutrients like nitrogen and phosphorus are less available.
- Above pH 7.5: Micronutrients like iron and manganese are harder for plants to absorb.
Adjusting soil pH can help unlock these nutrients, making fertilizers more effective and reducing waste. For acidic soils, adding lime can neutralize the acidity. For alkaline soils, adding sulfur can lower the pH and make nutrients more accessible.
Plants and Their pH Preferences
Different plants thrive in different pH levels:
- Acid-Loving Plants: Blueberries, azaleas, and rhododendrons prefer pH levels of 4.5 to 5.5.
- Neutral pH Plants: Tomatoes, beans, and lettuce grow best between 6.0 and 7.0.
- Alkaline-Tolerant Plants: Lavender and lilacs thrive in soil with a pH above 7.5.
Example: Managing pH in Auckland Gardens
A gardener in Auckland grew blueberries and lavender in the same yard. Blueberries needed acidic soil, while lavender thrived in alkaline soil. By creating separate beds and adjusting the pH for each, the gardener helped both plants grow successfully. This shows how managing soil pH can make a big difference.
How to Test Soil pH
Testing your soil’s pH is the first step in managing it. There are several ways to test:
- DIY Kits: Affordable and simple, these give you a general idea of your soil’s pH.
- Digital Meters: More accurate, these tools are great for gardeners who want precise measurements.
- Laboratory Testing: This is the most detailed option and can also provide information about nutrients and organic matter in your soil.
Tips for Testing
- Take samples from multiple spots in your garden to get an accurate reading.
- Test soil at a depth of about 6 inches, where most plant roots grow.
- Use clean tools to avoid contaminating your samples.
Adjusting Soil pH
Raising Soil pH (For Acidic Soil)
If your soil is too acidic, you can raise the pH by:
- Adding Lime: Ground limestone is the most common amendment. Dolomitic lime is a good choice if your soil also needs magnesium. Apply about 5 pounds per 100 square feet to raise the pH by one point.
- Using Wood Ash: This acts quickly but should be used sparingly to avoid overcorrecting the pH.
Lowering Soil pH (For Alkaline Soil)
To make soil more acidic, you can:
- Add Organic Matter: Materials like pine needles, peat moss, and compost help lower pH gradually.
- Apply Sulfur: Elemental sulfur is very effective but takes time to work, as microbes need to break it down.
Example: Blueberries in Bay of Plenty
A gardener in Bay of Plenty wanted to grow blueberries but had sandy soil with a pH of 7.8. By adding peat moss and sulfur, they lowered the pH to 6.5, which was ideal for blueberries. The plants thrived and produced a great harvest.
Compost and pH
Compost is a natural way to balance soil pH. It acts as a buffer, helping stabilize soil that’s too acidic or too alkaline. Compost also improves soil structure, water retention, and microbial diversity, all of which benefit plant growth. Regularly adding compost can help maintain healthy soil over time.
Advanced pH Management
For larger gardens or farms, consider these strategies:
- Crop Rotation: Growing different crops can naturally balance soil pH and nutrients.
- Cover Crops: Plants like clover and rye improve soil health and prevent erosion while helping balance pH.
- Precision Agriculture: Using tools like GPS and soil mapping, farmers can apply amendments only where needed, saving time and resources.
- Biochar: This carbon-rich material helps stabilize pH and improves soil quality.
Conclusion
Soil pH plays a big role in how plants grow. By testing and adjusting pH, you can help your plants absorb nutrients, grow stronger, and produce more. Whether you’re gardening at home or farming on a larger scale, understanding soil pH can make all the difference. With the right tools and techniques, you can create healthier soil and support sustainable gardening practices.